If unexpected leaks or trips to the bathroom frequently interrupt your day, you're not alone. Millions of men and women worldwide suffer from overactive bladder, stress urinary incontinence, and bowel incontinence. A life with fewer accidents is possible without the pads, pills, and planning. Tens of thousands of people have already found real relief with Axonics Therapy and Bulkamid.
1 in 6
adults experience Overactive Bladder (OAB)1
1 in 3
women experience Stress Urinary Incontinence (SUI)2
1 in 12
adults experience Fecal Incontinence (FI)3
Take the Quiz
to find out if Axonics Therapy or Bulkamid may be right for you.

Getting strong urges to urinate that are hard to control?

Experiencing leaks when you laugh, cough, sneeze, or exercise due to stress urinary incontinence (SUI)?

Not making it to the bathroom in time, and having accidental leakage?

Waking up several times a night to use the restroom?

Feeling sudden urges to have a bowel movement you are unable to control?

Planning your days around bathroom locations or packing extra pads to manage accidents?

Missing out on activities (like travel, dinner out, or hiking) due to fear of an accident?
If any of these sound like you, you’re not alone. People just like you have found real relief from bladder and bowel control issues with Axonics Therapy and Bulkamid.
 Take the first step toward real relief:
Take the first step toward real relief:
Take this short quiz to get started and see whether Axonics Therapy or Bulkamid may be right for you.

Axonics Therapy is a safe, user-friendly, clinically-proven therapy that uses Sacral Nerve Stimulation, also known as Sacral Neuromodulation, to help restore healthy communication between the brain, bladder and bowel. Patients just like you love Axonics Therapy because it:

of patients achieved clinically significant improvements4

of patients were satisfied with their therapy4

Before Axonics, many of my passions had been sidelined. Embracing my active life again has been a game-changer for me.
Jaime | Real Axonics Therapy PatientResults and experiences may vary and are unique to each patient.
I no longer have to live in isolation due to my condition. Axonics Therapy has been an absolute life changer for me and my family.
Ernest | Real Axonics Therapy PatientResults and experiences may vary and are unique to each patient.

With Axonics Therapy, I can confidently attend social events without the worry of unexpected accidents.
Tanzi | Real Axonics Therapy PatientResults and experiences may vary and are unique to each patient.

Bulkamid is a urethral bulking agent that is used to treat stress urinary incontinence (SUI). It is a soft, water-based gel that can be used to restore the natural closing of the urethra. Similar to some facial fillers it remains in the body over time without causing reactions in the surrounding tissue.1
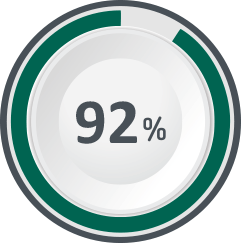
92% of women reported being cured or improved following treatment with Bulkamid.5

The majority of women with SUI choose Bulkamid before other treatment options.8

Before Bulkamid, I couldn’t play with my kids without worrying about leaks. But now, I have real relief.
Chelsea | Real Bulkamid PatientResults and experiences may vary and are unique to each patient.

I am no longer embarrassed or worried about accidents during exercise or even holding my grandkids after getting treated with Bulkamid.
Terri | Real Bulkamid PatientResults and experiences may vary and are unique to each patient.
Axonics Therapy is a clinically proven treatment that can help put you back in control of not just your bladder and bowel, but your life. It’s not a drug - it’s an advanced therapy that can provide lasting relief. If you’re ready to get your life back, it’s time to find real relief with Axonics Therapy.
 Take the first step toward real relief:
Take the first step toward real relief:
Take this short quiz to get started and see whether Axonics Therapy or Bulkamid may be right for you.
References:
IMPORTANT SAFETY INFORMATION:
Indications: Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence in patients who have failed or are not candidates for more conservative treatments.
Contraindications: Axonics SNM Therapy is contraindicated for patients who have not demonstrated an appropriate response to test stimulation; or Patients who are unable to operate the Axonics SNM Systems.
Warnings: Implantation and use of the Axonics Systems incur risks beyond those normally associated with surgery, some of which may necessitate surgical intervention. These risks include, but are not limited to adverse change in voiding function (bowel and/or bladder), infection, pain or irritation at the implant site, lead or device migration, electrical shock, change in sensation or magnitude of stimulation which has been described as uncomfortable (jolting or shocking) by some patients, and heating or burns at the device site.
For more safety information about indications and potential risks, go to www.axonics.com/isi.
Precautions: The safety and effectiveness of Axonics Therapy has not been established for use in women who are pregnant or in delivery; for pediatric patients (under the age of 18 years for fecal incontinence and under the age of 16 years for overactive bladder and urinary retention); for patients with neurological diseases, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: U.S. Federal law restricts this device to sale and use by, or on the order of, a physician.
For a complete listing of indications, contraindications, warnings and precautions, go to www.axonics.com/isi.

IMPORTANT SAFETY INFORMATION:
Indications: The Bulkamid Urethral Bulking System is indicated for urethral injection for the treatment of stress urinary incontinence (SUI) due to intrinsic sphincter deficiency (ISD) in adult women who have SUI or stress predominant mixed incontinence.
Contraindications: Bulkamid Urethral Bulking System must not be used in patients suffering from acute urinary tract infection.
Warnings: Do not inject Bulkamid Hydrogel intravascularly. Injection of Bulkamid Hydrogel into blood vessels may cause vascular occlusion leading to a possible embolism. Discontinue injection of Bulkamid Hydrogel if the superficial capillaries of the mucosa start to fade in order to avoid ischemia. Prior assessment of the tissue is recommended before introducing the Bulkamid Rotatable Sheath into the urethra. Do not force the Bulkamid Rotatable Sheath into the urethra or inject Bulkamid Hydrogel if the urethral tissue is damaged. The Bulkamid Urethral Bulking System should not be used in patients with urethral or bladder neck strictures until the strictures have been corrected. Use of the Bulkamid Urethral Bulking System in patients with strictures may cause injury and/or urethral obstruction. Over-correction using Bulkamid Hydrogel may lead to obstruction. Patients receiving treatment affecting blood coagulation have an increased risk of hematoma or urethral bleeding, as with any invasive procedure. Do not use Bulkamid Hydrogel in male patients.
Precautions: The Bulkamid Urethral Bulking System is only to be administered by a qualified physician, e.g. gynecologist, urologist, or urogynecologist. Safety and effectiveness of Bulkamid have not been established in patients with a fragile urethral mucosal lining, with urethral hypermobility with a straining angle >30º from horizontal bladder neck, predominant urge incontinence, detrusor overactivity, known polyuria (≥ 3L/24h), unevaluated hematuria, prolapse stage greater than Stage II using the ICS Pelvic Organ Prolapse Quantification (POPQ) exam, BMI >35 kg/m2, neurogenic bladder, less than 18 years of age, have active Herpes Genitalis, or for re-injection of Bulkamid Hydrogel less than 4 weeks after initial injection. The effect of Bulkamid has not been evaluated in women during pregnancy, delivery or lactation. The effect of Bulkamid on subsequent pregnancy and delivery, and the impact of subsequent pregnancy on the effect of Bulkamid, is unknown. Therefore, the risks and benefits of the device in women of childbearing potential should be carefully assessed.
Adverse Events: Adverse events may include: pain at the implant site, acute retention, urinary tract infection, hematuria, de novo urge incontinence, dysuria, urinary urgency, vaginal infection/irritation/Lichen Sclerosus, and worsening urinary incontinence.
Caution: U.S. Federal law restricts this device to sale and use by, or on the order of, a physician.
For a complete listing of indications, contraindications, warnings and precautions, go to www.bulkamid.com/isi.